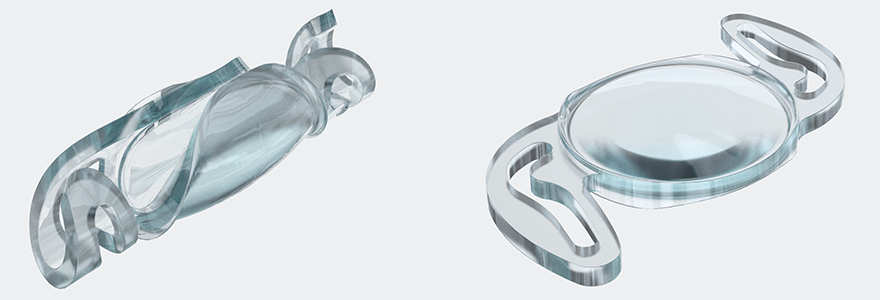
Eye Consultants of Texas founder and chief surgeon, Phillips Kirk Labor, MD is the first in North America to implant the Rayner RayOne EMV, a unique new premium intraocular lens (IOL) which received FDA approval in March 2021.
The RayOne EMV was developed by Rayner in collaboration with world-renowned eye surgeon, Professor Graham Barrett. “Rayner developed the world’s first IOL in 1949, which speaks highly to their innovation and credibility,” Dr. Labor said. “Knowing that someone with Professor Barrett’s credentials helped develop this lens, I couldn’t wait to learn about how it could significantly benefit my cataract patients.”
“EMV” stands for “Enhanced Monovision.” When configured as “mini-monovision” or “true monovision,” where lens power is specifically set for the dominant and non-dominant eyes, the RayOne EMV is designed to extend depth of focus for higher quality, uninterrupted vision throughout the entire intermediate and distance visual range with less dependency on glasses.
In addition, the RayOne EMV comes pre-loaded in an injector. What’s special about this is that it requires the world’s smallest incision for implantation in the eye. “A smaller incision reduces surgery and recovery time, which can improve a patient’s surgery outcome,” Dr. Labor added. “I’m happy to report that feedback from patients who have had the EMV implanted has been overwhelmingly positive.”
According to Rayner, the enhanced monovision and pre-loaded advantages makes the RayOne EMV the only FDA-approved lens of its kind in the world. Dr. Labor was not only the first North American surgeon to implant this lens, he is among a select few U.S. eye surgeons Rayner consults with on this lens, starting with helping ensure its effectiveness for FDA approval.